Spectrometry Lab - Live Component
Introduction:
The purpose of this lab is to learn the methods that scientists use to determine the basic chemical composition of objects in our universe. We will observe and study different types of gases and elements and identify those elements based upon their spectral emissions.
We used a simple spectrometer to look at and see the spectra of different elements, objects, and types of light. We used a variety of light sources and objects, such as an incandescent light bulb to study and test the spectra of.
Procedures:
Part I: Observing Spectra using a Diffraction Grating
- A. I was provided with a spectrometer with which to observe spectra. The most important part of the spectrometer is called a diffraction grating. The grating in the spectrometer is used to disperse the light into a spectrum for viewing on the spectrometer scale is used to disperse the light into a spectrum for viewing on the spectrometer scale. Before using the spectrometer, I studied the 2”x2” diffraction grating found in my lab packet. This sample grating is similar to the one in the spectrometer. I noticed bright colors seen through the grating and also reflected off its surface. A grating uses the wave phenomena of diffraction and interference to produce spectra. A prism, on the other hand, uses the fact that different colors of light are refracted at different angles as they pass from air through glass.
- B. Holding the grating close to one eye and looking through it toward an incandescent light bulb, I saw a continuous spectrum of color off to either side of the bulb. I had to shift my head around somewhat to see these. I described what I saw, and payed careful attention to the order of the colors and locations compared to the light bulb.
- C. Using the spectrometer: The remainder of the exercise I did using the spectrometer. I looked through the diffraction grating at the narrow end of the device. I noticed that there is a narrow “slit”, opposite the diffraction grating. I held the end with the diffraction grating near my eye. As I looked through the grating, I found the slit. Holding the spectrometer steady near my eye, I twisted my upper body slowly horizontally, left and right, until I saw light from a source. Without moving my body, I glanced to the right of the interior of the spectrometer and saw the spectrum displayed on the scale. I used the wavelength scale that is labeled 700 through 400 running from left to right. These are wavelength values in units of nanometers (where 1 nm = 10^=9 m). I noted that the corresponding range in units of Angstroms would be from 7000 through 4000.
- I looked at the spectrum of a light bulb through the spectrometer. I saw all the colors of the spectrum aligned with the wavelength scale. When I used my calibrated spectrometer to observe the spectrum, I noticed that each color falls on a different portion of the wavelength scale. I described what I saw, and what colors correspond to what wavelengths.
- I looked carefully at the left edge of the spectrum and noted the wavelength scale reading at which the light disappears. I did the same on the right edge of the spectrum. These are the wavelengths "limits" of sensitivity of my eye. I recorded these wavelength numbers.
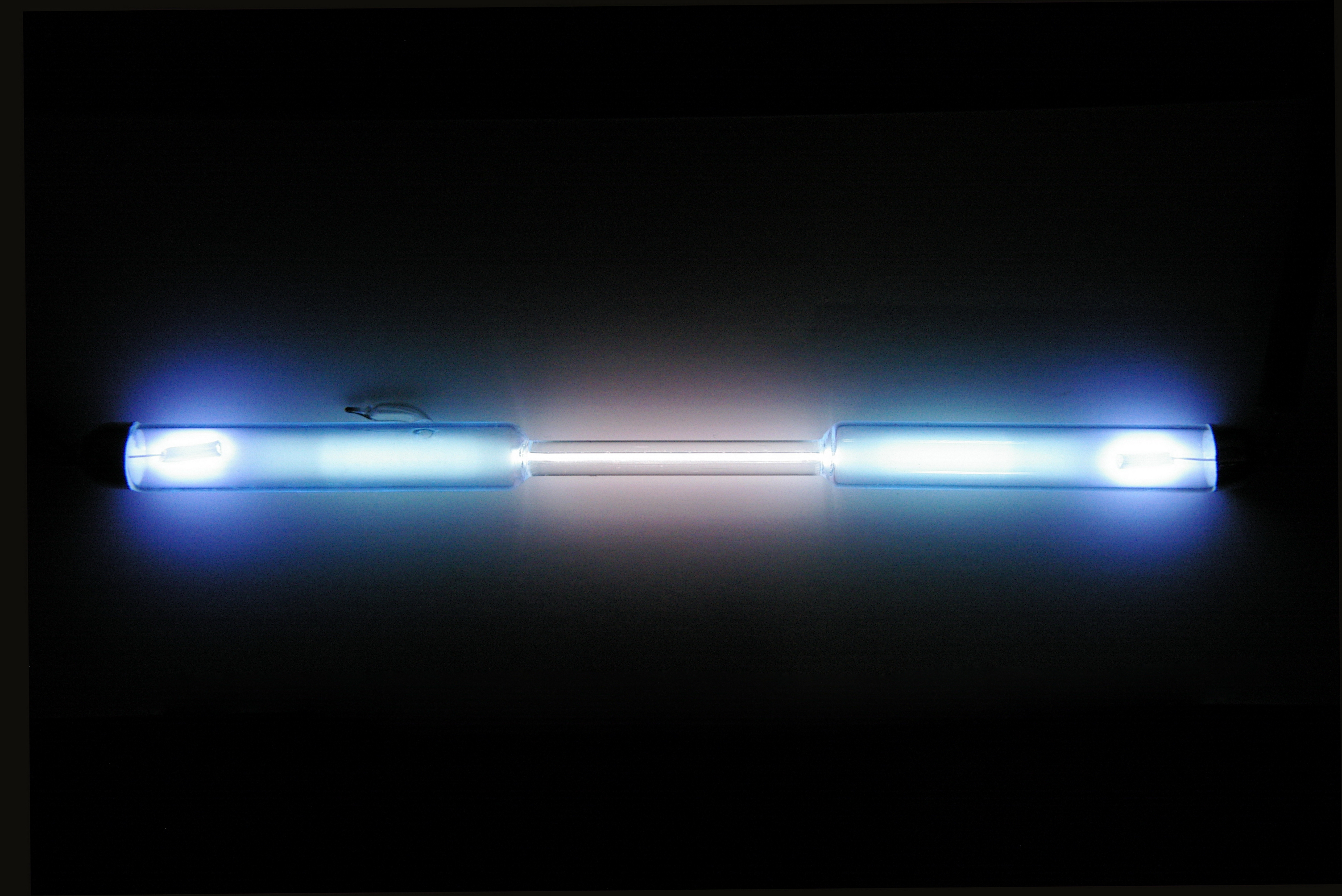
- In this section, I looked at several spectrum tubes, each filled with the gas of a specific chemical element. When a high voltage is applied to the ends of the spectrum tube, current flows through the gas and heats it. A hot gas emits only certain colors of light. Each type of gas produces a unique pattern of bright spectral lines. As I viewed each tube with my spectrometer, I noted the wavelengths of dominant bright lines of each element. I described what I saw. I also noted the general color of the tube of gas as seen with an un-aided eye in my blog post. Hot gasses do not produce a continuous band of colors, and the unaided eye will see only one combined color, which will be a mix of all the different colors in the spectrum of a particular element. I used this information to identify the unlabeled gases. I studied the emission spectra of hydrogen, helium, mercury, neon, and sodium.
- I looked at a florescent light bulb with my spectrometer. I noted that it appears to have a continuous spectrum with emission lines on top of it. I recorded the wavelengths that I observed.
- Then I observed two other light sources of my choice.
- I compared these spectra to my previous results. I tried to determine which gases are in these lights by the kind of spectra they had and by the patterns of the spectral lines that they produced.
Results and Discussion:
1.) Incandescent Light bulb:
2.) Guess: Hydrogen
4.) Guess: Krypton
5.) Guess: Helium
1.) Incandescent Light bulb:
- Observations: The spectra of the incandescent light bulb was a continuous lone of every color of the rainbow.
2.) Guess: Hydrogen
- Observations: The color of the bulb was purple.
- Purple = 490
- Red = 655
- Orange = 590
3.) Guess: Neon
- Observations: The color of the bulb was red.
- Red = 610, 650, 670
- Orange = 600
- Yellow = 580
- Green = 540
- Blue=510
4.) Guess: Krypton
- Observations: The color of the bulb was a dim light blue.
- Orange: 590
- Green = 550
- Teal = 510
- Purple = 400
- Observations: The color of the bulb was a yellowish-pink.
- Red = 610
- Yellow = 580
- Teal = 500
- Blue = 420
- Observations: The color of the bulb was pink.
- Yellow = 580
- Green = 540
- Blue - 440
1.) Pink rain boot:
- Observation: brightest on the spectrum at red, purple, and green; not very visible at blue and yellow.
Conclusion:
Using the spectrometer, I was able to determine the different elements by observing each spectra. I now have the knowledge and skills to understand how astronomers determine the spectra of objects in outer space.
Using the spectrometer, I was able to determine the different elements by observing each spectra. I now have the knowledge and skills to understand how astronomers determine the spectra of objects in outer space.






No comments:
Post a Comment